We just wished to extend our sincere thanks to the Olon team for such excellent support over the past number of years. It is very much appreciated.
Olon Regulatory Affairs Department is a major asset and it has seen significant development and diversification of its expertise in terms of regulatory frameworks and requirements of different geographic areas, including the most complex and demanding ones. We hold in-depth knowledge of the worldwide regulatory processes, including Countries as China, Brazil and Japan ─ expertise gained from decades of consolidated, hands-on experience.
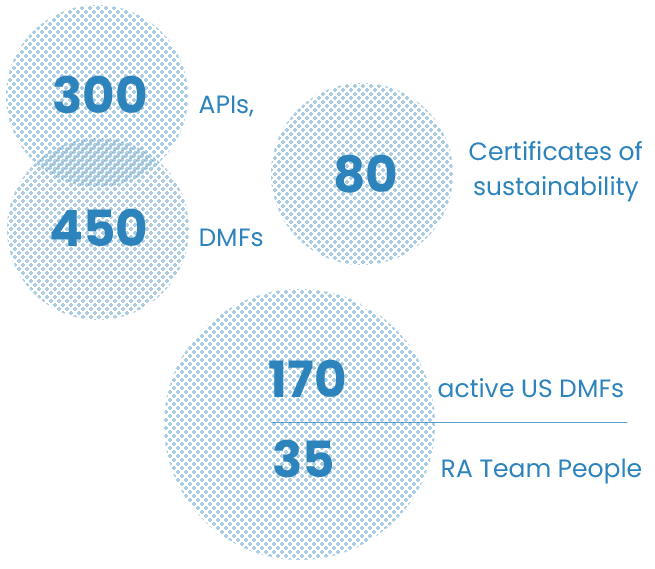
Our drivers are strategic consultancy, transparency, and continuous feedback to our customers for both generic and CDMO projects. Olon RA team comprises 35 highly qualified professionals located in Europe and Asia.
With as many as successfully submitted worldwide in support of our customers’ marketing authorizations and clinical trials, with its diverse, in-depth knowledge of international regulatory frameworks, Olon regulatory affairs service represents an excellence in the API industry.