Olon Group is a global leader in the development and production of active pharmaceutical ingredients (APIs) for CDMO and generic markets, integrating chemical synthesis and biological processes while always embracing the highest international safety, quality, and environmental standards.
With one of the longest track records of the API industry, having deep development expertise and a broad set of advanced technologies, we are the partner of choice which enables our client’s molecules to enter the market successfully
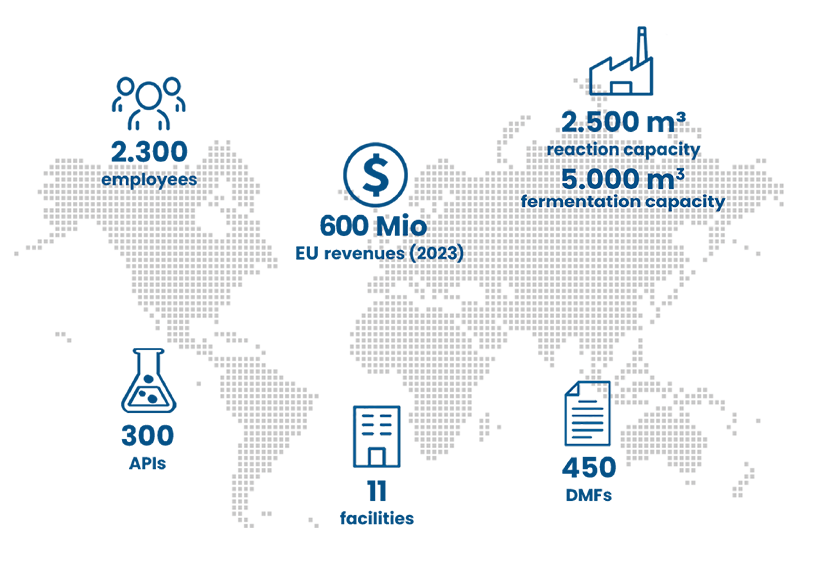
Olon has a global network of 11 manufacturing sites and 7 R&D centers across the globe. Thanks to our 2,300 employees, including 300 highly experienced and qualified R&D experts, we represent a highly innovative and reliable partner
At Olon, expertise and competent flexibility throughout the organization help build successful outcomes for our clients in custom chemical synthesis and microbial fermentation, while always maintaining the highest levels of safety, quality, and environmental compliance.
With our structured expertise in chemical synthesis and microbial biomanufacturing, we are one of the leading international players of API and HPAPI production. Our sites are equipped with multi-purpose, dedicated lines where we can produce at different levels of high containment, up to ultra-high-containment for high-potency and toxic products.
Our expertise is applied to the production of APIS for treatments used in many key therapeutic areas and for many breakthrough pharmaceutical products. We are leaders in the production of retinoids, antineoplastic, cardiovascular and metabolic diseases compounds, antibiotics and antivirals.